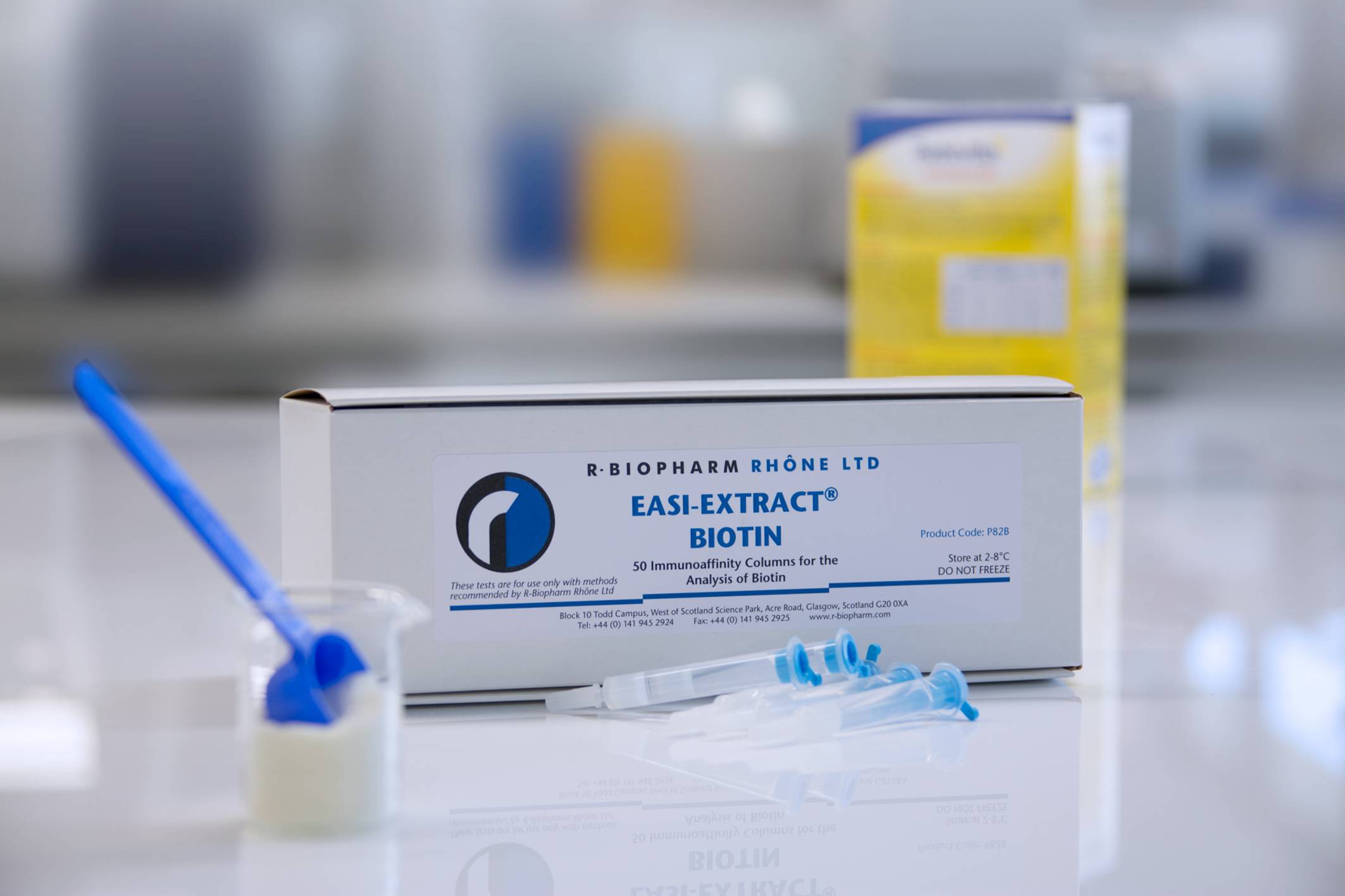
Description
Intended use:
Immunoaffinity columns for use in conjuntion with an HPLC or LC-MS/MS system for detection of biotin in a wide range of commodities.
General Information:
The procedure is based on monoclonal antibody technology, which makes the test highly specific, sensitive, rapid and simple to perform. The columns contain a gel suspension of monoclonal antibody specific to the vitamin of interest. Following extraction of the vitamin the sample extract is diluted with buffer and filtered before being passed slowly through the immunoaffinity column. Any vitamin which is present in the sample is retained by the antibody within the gel suspension. The column is washed to remove any unbound material and the vitamin is then released from the column following elution with solvent. The eluate is collected, evaporated and reconstituted prior to analysis by HPLC or LC-MS/MS.
Benefits:
- AOAC Official Final Action method 2016.02.
- Uses monoclonal antibody.
- Extraction uses buffer and enzymes.
- Can be used with pigmented samples.
- Can be stored at 21 – 25 °C.
- The columns are suitable for testing a wide range of commodities.
Accessories:
Certificates:
| Article Numbers | RBRP82 / RBRP82B |
|---|---|
| Test format | RBRP82 = 10 immunoaffinity columns with 3 ml format. RBRP82B = 50 immunoaffinity columns with 3 ml format. |
| Sample preparation | Phosphate buffer, pancreatin, sodium ascorbate solution |
| Incubation time | 3 hours prior to detection by HPLC or LC-MS/MS |
| LOD (Detection limit) | 25 ng / ml |
| Validated matrices | Infant formula, ready-to-use infant formula, infant cereal, liquid milk |
| Available application notes | Beer, energy bars, health supplements |
| Detected analyte | Biotin |
| Evaluation | HPLC or LC-MS/MS system |
| Approvals | Received AOAC Official Final Action Method 2016.02. |
| Instructions | |
|---|---|
| MSDS |