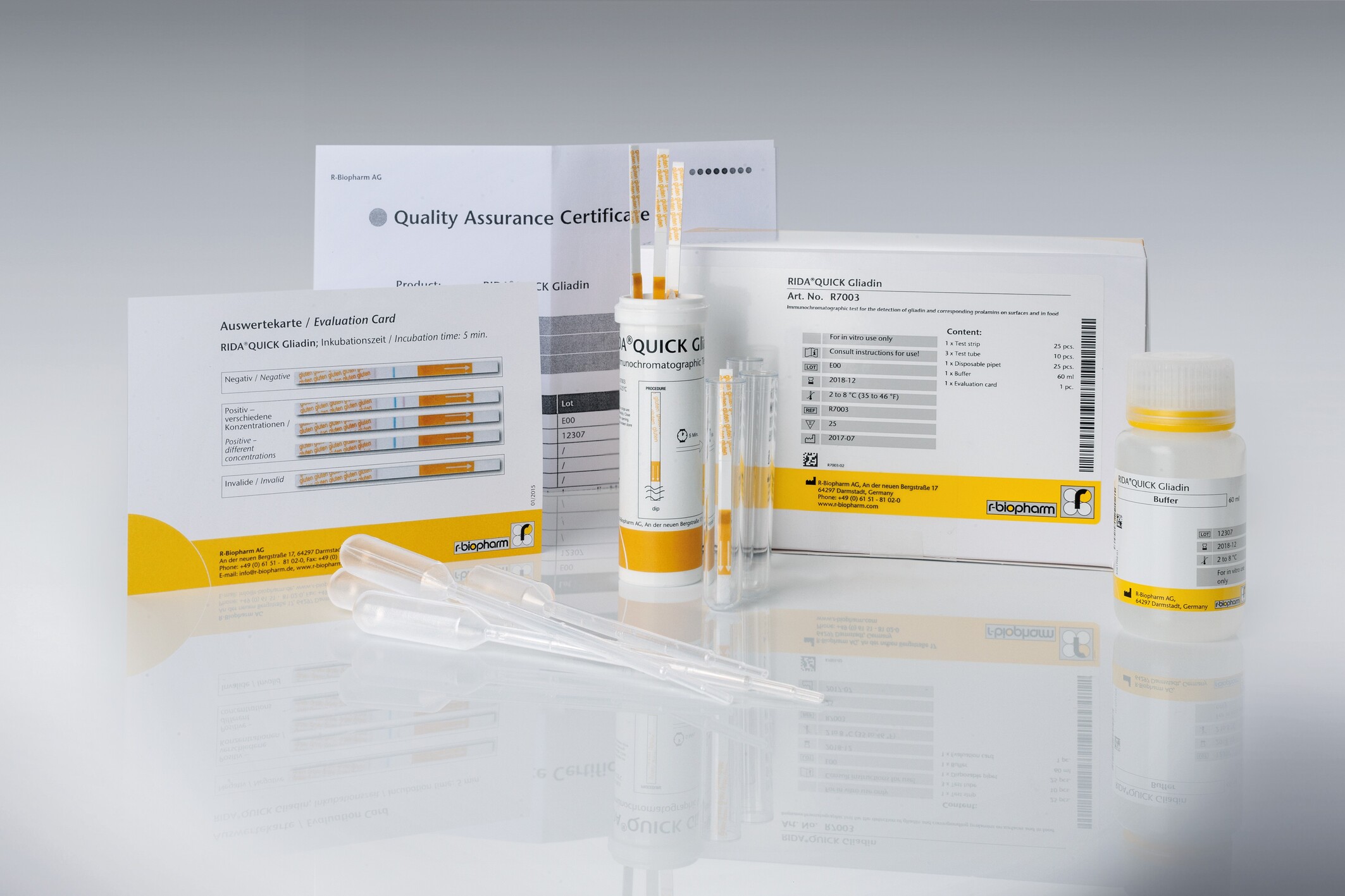
Description
Intended use:
RIDA®QUICK Gliadin can be used as a swab test for gluten detection on surfaces in the hygiene control, in cleansing waters, and for the qualitative detection of gliadin / gluten in raw material and processed food. The test has been developed for the detection of low amounts of gluten (contamination). No high-dose-hook-effect is observed at high concentrations.
General Information:
The use of wheat flour and gluten in foodstuff is extremely common because of their useful effects on e.g. texture, moisture retention and flavour. Gluten is a mixture of prolamin and glutelin proteins present in wheat, rye and barley. Coeliac disease is a permanent intolerance to gluten that results in damage to the small intestine and is reversible when gluten is avoided by diet.
The official type I method for gluten determination according to the Codex Alimentarius is an ELISA which uses the R5 antibody (Mendez). This requirement is fulfilled by RIDASCREEN® Gliadin test (R7001). The RIDA®QUICK Gliadin test strips also use the R5 antibody and show a good correlation with the official method, the R5-ELIS A RIDASCREEN® Gliadin. R-Biopharm AG is the only company, that is allowed to use the R5 antibody for the test strips.
You can find further information on the scientific background of the test system in the following documents:
- Validation of a qualitative R5 dip-stick for gluten detection with a new mathematical-statistical approach. View article
- Determination of Gluten in Processed and Nonprocessed Corn Products by Qualitative R5 Immunochromatographic Dipstick: Collaborative Study, First Action 2015.16. View article
- The Validation of the RIDA®QUICK Gliadin for AOAC Research Institute. View article
Approvals:
- Surfaces and in clean-in-place waters: The dip stick RIDA®QUICK Gliadin was approved by the AOAC Performance Tested MethodsSM Program and was assigned PTM Certification No. 101702 by the AOAC Research Institute. The in-house validation data was confirmed by an independent lab and demonstrated that the dip stick is applicable for the detection of traces of gliadin on surfaces (stainless steel, sealed ceramic, plastic and silicone rubber) and in clean-in-place waters.
- Food samples: The RIDA®QUICK Gliadin test kit was also evaluated by 18 labs in an international collaborative study for food samples. The immunochromatographic test is an AACC international approved method (38-60.01) and an AOAC approved Official Method of Analysis (Final Action OMA 2015.16).
Watch our video tutorials for swabbing and test procedure with RIDA®QUICK Gliadin:
Accessories:
Certificates:
| Article Numbers | R7003 |
|---|---|
| Test format | 25 x test strips in reclosable tube for 25 determinations |
| Incubation time | 5 min |
| LOD (Detection limit) | – Surfaces approx. 1.6 – 3 μg gluten / 100 cm2 – Raw material approx. 4.4 mg / kg gluten – processed food approx. 6.3 mg / kg gluten – Cleansing water (without cleansing reagent) approx. 10 ng / ml gluten – Cleansing water (with cleansing reagent) approx. 50 – 100 ng / ml gluten |
| Available application notes |
|
| Evaluation | Visual evaluation |
| Instructions | |
|---|---|
| MSDS |